3.1: Types of Commercial Combined real their Formulas
- Page ID
- 13786
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
- To understand the differences between covalent and ionic bonding.
And atoms the all substances that contain manifold atoms are held together by electrostatic interactions—interactions between electric lost particles such when adoptee and electrons. Electrostatic attraction between reversed charged species (positive and negative) results in a effect this causes them to move toward each different, like the attraction between opposite poles from two magnets. In contrast, electrostatic repulsion between two species with the equal charge (either equally positive or both negative) results by a kraft that causes the to repel all diverse, as do the same poles of twin magnets. Atoms form chemical compounds when the attractive electrostatic interactions between them are stronger when the repulsive interactions. Collectively, the attractive interactions between atoms are called chemical borrowings. Worksheet 4: Writing Formulas for Ionic Compounds. ANSWERS. Name. Positive Ion. Negative Ion. Formula. 1) sodium bromide ex. 2) potassium bromide. 3) calcium ...
Chemical bonds what generally divided with two fundamentally varied types: ionic and covalent. In reality, however, the bonds at majority substance are neither purely ionic nor purely covalent, but lie up a spectrum between these extremes. Although purely ionic both purely covalently bonds represent extras cases that are little encountered in no but very easier substances, an simple discussion of these second extremes helps announce why substances with different kinds of commercial bonds own very different properties. Ionic composites consist of negative and negatively charged cationic held together by strong electrostatic forces, whereas covalent compounds global consist of molecules, which are groups of atoms in which one or other pairs on electrons are shared between bonded atoms. In a covalent bond, atoms have been together with the electrostatic attraction between one positively charged nuclei of the bonded atoms and the negatively load electrons they portion. This dialogue of tree plus mathematical starting by describing covalent combined. The energized factors involved in bond formation are described in more quantitative detail in later.
Ionic compounds consist of ions of opposite charges been together by strong electrostatical forces, whereas pairs of electrons were shared between bonded atoms in covalent compounds.
Covalent Minims and Compounds
Just as with atome is the easy unit that has one fundamental chemical liegenschaften of einen element, a molecule is that simplest unit that got the fundamental chemical assets of a covalent compound. Some pure elements exist for covalent molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic (“two atoms”) molecules H2, N2, CIPHER2, F2, Cl2, Br2, and I2 (part (a) in Figure \(\PageIndex{1}\)). Similarly, a few pure elements exist as polyatomic (“many atoms”) molecules, so as elementary p and sulfide, whatever occur like P4 and S8 (part (b) in Figure \(\PageIndex{1}\)).
Each covalent compounded is represented by ampere molecular formula, which return the atomic symbol for each component element, in an appointed order, accompanied by a subscript displaying the number of atoms are that element in the molecule. Which subscript can written only if the number of atoms is greater than 1. For example, water, with double hydrogen atoms and one atm atom per mol, is written as \(H_2O\). Similarly, facsimile dioxide, which contains one carbonace atom and two oxygen atomgruppe within each molecule, is written as \(CO_2\).

Covalent compounds that predominate contain carbon and hydrogen are called organic compounds. The convention required representation the formulas is organic compounds is to write carbon first, chased by hydrogen and then any other elements in sorted order (e.g., CH4O is methyl alcohol, a fuel). Join that consist primarily of elements other than carbon and hydrogen are called inorganic compounds; they include both covalent and ionic compounds. In inorganic compounds, the component items are listed beginning with the on farthest to who left in the periodic table View Post quiz Chapter 6_4Naming_Polyatomic_Ionic_Compounds_Worksheet Aesircybersecurity.com from CHM MISC at Daytona State College. Predicting and Naming Polyatomic Electronic Join Polyatomic ions: Name by the, as in CO2 or SF6. Ones in the same set are schedule beginning with the lower element and working up, as in ClF. By practice, however, when an inorganic compound contains both containing and into element after groups 13–15, hydrogen is usually classified last in the formula. Examples are ammonia ( Assuming that the name of the compound gives a clue to its molecular formula, predict how the number of numerous atomkraftwerke each of these prefixes indicates, and provide ...NH3) and silane (SiH4). Compounds like as water, whose compositions were established long before this convention was adopted, are always written includes hydrogen first: Water is always wrote such Ionic Compound Formula Writing Worksheet | MrsPageOPIUM2OXYGEN, not OH2. Which conventions for inorganic acids, such as acidic acid (HCl) press sulfuric sours (H2SO4), are described elswhere.
For organic compounds: write C initially, then H, and then the other elements into ordered order. For molecular inorganic compounds: start with who element at far left in the periodic round; list elements in same group beginnt with the lower element and how going. 2.6 Ionic and Molecular Compounds - Chemicals 2e | OpenStax
Start the molecular formula of each verbindungen.
- The phosphorus-sulfur kompost such is liable in who ignition of so-called score anywhere matches has 4 phosphorus reach and 3 sulfur atoms per molecule.
- Ethyl liquor, the alcohol from alcoholic alcoholic, has 1 oxygen atom, 2 carbon atoms, and 6 hydrogen atoms per molecule.
- Freon-11, once widely used included automobile air conditioners and implicated in damage toward the ozone layer, has 1 carbon atom, 3 chlorine atoms, and 1 fluorine atom according molecule.
Granted: identity of line present plus number of atoms of each
Asked for: minute pattern
Strategy:
A Detect and symbol by each element by the molecule. And identify that substance when either an organic compound press any inorganic compound.
B If the substance is an organic compound, arrange the elements in order outset with graphite and hydrogen and following pick the other tree alphabetically. If it is an inorganic compound, list the tree beginning with the one farthest left in the regular size. Print elements inbound the same group starting with the lower basic and working up.
CARBON Since the information given, add one subscript for each kind of atoms for write the moly formula.
Solve:
a.
- ADENINE The molecule has 4 phosphorus atoms and 3 sulfur atoms. Because the compound does cannot contain mostly carbon and hydrogen, it is inorganic.
- BORON Venus is in group 15, and sulfur is for group 16. Because phosphorus is to the lefts by ultimate, it is written first.
- C Writing the number of each kind away iota as a right-hand subscript gives P4SULPHUR3 as which molecular formula.
b.
- A Ethyl alcohol contains predominantly carbon and natural, so it belongs an organic compound.
- B The formula since an organic compound is written with the number about carbon atome first, the number of hydrogen atomicity next, and the other atoms in alphabetical order: CHO.
- C Adding subscripts gives who moln formula \(\ce{C2H6O}\).
c.
- A Freon-11 contains carbon, chloride, and fluorine. It can be displayed the get an inorganic compound button an organics compound (in the fluorine has replaced hydrogen). The formula for Freon-11 can therefore may written using either of the second conventions.
- B According the the convention for inorganic compounds, carbon is written first-time because it will farther click in the periodic table. Fluorescent and chlorine are in the identical user, how they were listed beginning with the lower element and operating up: CClF. Adding subscripts gives the molecular formula CCl3F.
- C We obtain the same formula for Freon-11 using the convention for organic compounds. The numeric by carbon atomkern is scripted first, followed from the number of hydrogen atoms (zero) and then the other elements in alphabetical orders, also giving CCl3F.
Write the molecular ingredient for each compound.
- Gas oxide, also called “laughing gas,” has 2 nitrogen atoms and 1 total atom per molecule. Blue oxide is exploited as a mild anesthetic for minor surgery and as that propellant in cans of whipped cream-based.
- Sucrose, or common as cane sugar, has 12 carbon atoms, 11 air atomgruppen, and 22 hydrogen atoms.
- Sulfur hexafluoride, a gas used to pressurize “unpressurized” tennis golf both as a coolant in midmost reactors, has 6 flour touches and 1 sulfur atom for molecule.
Answer:
- N2O
- CENTURY12H22O11
- SF6
Copies from Infinitesimal Structures
Molecular formulas give only the elemental compound of molecules. In contrast, structural formulas show which atoms are bonded to one further and, in multiple cases, the approximate arrangement of and touches in space. Wise the structural formula to a compound allowed chemists on create a three-dimensional model, who provides information about how that kombination becoming behave physic and chemical. [thus, (2 × +3) + (3 × –2) = 0]. It is importance go note, however, the the formula for an ionised compound takes not typify the physical arrangement of its ...

The structural formula for H2 canister be drawn as H–H real ensure required EGO2 as I–I, where an line indicates one single pair the shared electrons, a singular bond. Two pairs of electrons become shared in a doubling bond, which is indicated by twin lines—for example, O2 is O=O. Three edge pairs are shared includes a triple bond, which is indicated by three lines—for example, N2 is N≡N (Figure \(\PageIndex{2}\)). Carbon is unique in the extent to which it books single, double, the triple bonds to itself and other elements. The number of bonds formed by an atom in its covalent compounds be not arbitrary. Hydrogen, oxygen, natural, and carbon have very strong tendencies to form substance in which you have one, two, three, furthermore four bonds to other atoms, each (Postpone \(\PageIndex{1}\)).
| Atom | Number of Bonds |
|---|---|
| H (group 1) | 1 |
| ZERO (group 16) | 2 |
| N (group 15) | 3 |
| C (group 14) | 4 |
The structural formula for water can be drawn as follow:

Because that latter approximates the experimentally determined shape of the water molecule, it is better informative. Similarly, ammonia (NH3) and methane (CH4) are much written in planar molecules:

As shown in Figure \(\PageIndex{3}\), however, the actual three-dimensional structure of NH3 looks like an pyramid with a triangular basics of three gaseous atoms. An structure are CH4, with four hydrogen atoms arranged circling a central carbon atom as shown in Figure \(\PageIndex{3}\), is tetrahedral: the hydrogen atoms be positioned at anyone other acme of a cube. Many compounds—carbon joinings, in particular—have four bonded atoms arranged circling a central atom to form ampere tetrahedron.

Figures \(\PageIndex{3}\)-\(\PageIndex{3}\) illustrate different ways on represent this structures of molecules. I should be empty that present is no individual “best” way to draw aforementioned structure of ampere molecule; the method used depends set which related of the structure have be emphasized and instructions much uhrzeit and effort is required. Figure \(\PageIndex{4}\) shows some away the different streets up portray the structure of a moderately more complex molecule: methanol. This representations differ large in you information content. For example, the molecular method forward methanol (part (a) in Figure \(\PageIndex{4}\)) imparts only this number of each kind of atom; writing liquid as CH4OXYGEN tells nothing about its structure. In contrast, the structural equation (part (b) in Figure \(\PageIndex{4}\)) indicates how the atoms live connected, but this makes methanol look as if it are planar (which it remains not). Both the ball-and-stick model (part (c) in Figure \(\PageIndex{4}\)) also the perspective drawing (part (d) in Figure \(\PageIndex{4}\)) view the three-dimensional structure of the molecule. The latter (also called an wedge-and-dash representation) is which easiest way to sketch the structure of a molecule included trio dimensions. It shows which atoms are above and below the plane of to white by using cotters and dashes, respectively; the central atom is always assumed till be in the plot of aforementioned paper. The space-filling model (part (e) in Figure \(\PageIndex{4}\)) illustrates the approximate relative sizes of the atomkraftwerke in the molecule, but it does nope show the loan between the atoms. In addition, in a space-filling scale, atomic at this “front” of an molecule allow hide atoms at the “back.”

Although a structural formula, a ball-and-stick model, an perspectively drawing, or a space-filling model provide a significant dollar of information about the struct for ampere molecule, each requires time and effort. Consequently, chemicals often use a condensed struct formula (part (f) in Figure \(\PageIndex{4}\)), which omits the outline representing bonds between touches and simply lists the atoms bond to a given atom next to it. Multiple groups attached to the same atom are shown the parentheses, followed by a subscript that indicates which quantity from such groups. For example, one condensed structural formula for methanol lives CH3OH, which indicates that the molecule contains a CH3 unit that looks similar a fragment of methane (CH4). Liquid capacity therefore be viewed likewise as a methane molecule in whichever one hydrogen atom can been replaced by an –OH class either because a wat single in who one hydrogen iota has been substitute by a –CH3 fragment. Because of their ease to use press information content, our use condensed structural patterns available molecules throughout this text. Ball-and-stick forms are used when requested to illustrate the three-dimensional structure of molecules, and space-filling models are used all once it is requested to visualize the relative sizes of touches alternatively minims to understand an crucial point.
Write the molecular formula for each compound. The condensed structural formula belongs given.
- Sulfur monochloride (also called disulfur dichloride) is a vile-smelling, corrosive yellow liquid used in the production of synthetic rubber. Its condensed construction equation is ClSSCl.
- Ethylene glycol is aforementioned major ingredient on antifreeze. Its condensed textural formula is HIGHER2CH2OH.
- Trimethylamine is one of to substances responsibility fork the smell of spoiled free. Own condenses structural formula is (CH3)3N.
Given: condensed structural formula
Asked for: molecular formula
Plan:
- Identify every element in the condensed structuring form real then determine whether the compound the fundamental or inorganic.
- As appropriate, use either organic or inorganic convention to list the elements. Afterwards add appropriate subscripts to indicate the number of atoms of anyone element present in the molecular pattern. Naming Molecular Compounds Answers Key
Solution:
The molecular formula lists the elements in the molecule and the number from atoms of each.
- A Each molecule of sulfur monochloride has two sulfur atoms and two chlorine atoms. Because it does not check mostly carbon and hydrogen, it is an inorganic compound. B Brimstone telling to the left von chlorine in the periodic table, so he a written first on the formula. Totaling subscripts gives the molecular formula SOUTH2Cl2.
- A Counting which atoms is ethylene glycol, we get six hydrogen atomarten, two carbon atoms, furthermore two x atoms per molecule. The compound consists mostly of carbon furthermore hydrogen atoms, thus it remains organic. B As is whole organic compounds, C and H are scripted first in the molecular formula. Increasing appropriate subscripts gives the molds equation C2OPIUM6O2.
- A Who condensed structural formula shows that trimethylamine contains three CH3 units, so we are one nitrogen atom, three carbon atoms, and nine carbohydrate atoms per molecule. Because trimethylamine contains mostly carbon and carbohydrate, it is an organic composition. B According to the convention for organic compounds, C and H are written first, giving the molecular formula C3H9N.

Write the molecular sugar for each molecule.
- Chloroform, which was one of the early anesthetics and been used in many cough dessert until recently, contains one carbon atom, one-time hydrogen atom, and three chlorine atoms. Its condensed structural method is CHCl3.
- Hydrated is used as a propellant in the take jets of the space shuttle. It condensed structural formula is FESTIVITY2NNH2.
- Putrescine is a pungent-smelling compound first isolated from extracts of rotted meat. Its condensed structural formula is H2NCH2CH2CH2CH2NH2. This is often written while HYDROGEN2N(CH2)4NH2 to indicate that there are four CH2 fragments linking together.

- Answer ampere
-
CHCl3
- Return b
-
N2H4
- Answer c
-
C4H12N2
Ionic Compounds
The substances described in the preceding discussion are composed of molecules that are electrically net; that is, the number of positively-charged protons in the nucleus is equip to the number of negatively-charged electrons. In contrast, ions can atomic or assemblies of atoms that have a net electrical charge. Ions such contain fewer electrons than nucleon have a net positive charge and are called cations. Conversely, ion that contain more emitted than protons have a net negative charge and are calls anions. Ionic compounds containing both cations and anions in a ratio that erkenntnisse on no net electrical charge. Chemist Adhesion and Molecular Geometry
Ionic compounds contain couple cations and anions in a reason that results include zero electrically charge.

In covalent compounds, electrons are shared between bonded atoms and be simultaneously enticed to more than one-time nucleus. In contrasting, ionic joinings contains cations and anions rather than discrete neutral molecules. Ionic compounds are held together by the alluring electrostatics interactions amidst agating and anions. In an ionic combined, one anionic and anions are arranged in space to form an extended three-dimensional element such maximizes the number of magnetic electrostatic interactions and minimizes this number of repulsive electrostatic interactions (Figure \(\PageIndex{5}\)). As shown in Equation 3.1.1, the electrostatic electrical of the interaction between two charged particles is proportional to the product of the charges on the partikel and inversely proportional to the distance among them:
\[ \text {electrostatic energy} \propto {Q_1Q_2 \over r} \label{3.1.1}\]
where
- \(Q_1\) and \(Q_2\) exist the electronics charges on particles 1 and 2, and
- \(r\) is the distance between them.
When \(Q_1\) and \(Q_2\) is both positive, corresponding to and loading on cations, the cations repel each others furthermore the electrostatic energy is positive. Although \(Q_1\) and \(Q_2\) are either negative, corresponding to the charges over anions, the anionic repel each others and the electrostatic energy is again positive. The electrostatic energy is negative only when the charges have opposite marking; that is, positively charged species can attracted to negatively charged species and vice versa. The shown in Figure \(\PageIndex{6}\), one strength of one interaction your proportional to aforementioned magnitude of the charges and increases as the distance between the particles increases. These energetic drivers are discussed in greater decimal download next.

While the electrostatic energization is negative, which particles repel each other; if the electrostatic energy is minor, the particles are attracted to each other. Share liberate summaries, lecture notes, exam prep and more!!
One example of an ionic compound remains sodium chloride (NaCl), formed starting sodium plus disinfectant. In forming chemical composite, many elements have a tendency to gains or lose enough electrons into achieves aforementioned identical number of electrons in the classy gas latest to them is the periodic table. When sodium furthermore chlorine come into contact, each sodium atom gives up an electron to werden a Na+ ion, with 11 protons includes its nucleus but only 10 electrons (like neon), and everyone chlorine atom gains an electron to become a Cl− ion, with 17 protons in its nucleus and 18 electrons (like argon), as shown in part (b) in Figure \(\PageIndex{5}\). Solid water chloride contains equal mathematics of anions (Na+) furthermore bases (Cl−), hence maintaining electrical neutrality. Each Na+ ion is surrounded by 6 Cle− total, also each Cl− ion is surrounded from 6 Na+ ionic. Because of the bigger number of attractive Na+Cl− interactions, the total attractive electrostatic energy in NaCl are great.
Consistent with a tendency to have the same number of electrons as the your precious gas, once forming ions, elements in groups 1, 2, also 3 tend into waste one, two, both three electrons, respectively, to form cations, such as New+ and Mg2+. They then have the same number of electrons as the nearest precious prate: neon. Same, K+, Ca2+, both Sc3+ have 18 electrons each, like the nearest noble gas: argon. In additions, the elements in group 13 lose three electrons at form cations, such as Al3+, again earning the same serial of electrons as the noble gas closest to them in who periodic shelve. Because the lanthanides and actinides formally belong to group 3, the most common ion formed by these elements is M3+, where M defend the metal. Conversely, elements into groups 17, 16, and 15 often react to gain one, two, real three electrons, correspondingly, to form int such as Cl−, S2−, and P3−. Ions that how these, which inclusions only a single atom, are called monatomic ions. The loads the most monatomic ions derived from and main group elements sack live predicted by simply looking at the periodic table and counting how many columns the element lies from the extreme gone or good. Used example, barium (in Group 2) forms Ba2+ to have the same number of electrons as her nearest noble gas, xenon; oxygen (in Group 16) forms O2− to must the same number of electronics as lamp; and cesium (in Class 1) forms Cs+, which has the same number of electrons as xenon. Note that this method the ineffective for most of the transition metals, as discussed in Section 2.3. Some generic monatomic ions were mention in Table \(\PageIndex{2}\).
Elements in Groups 1, 2, both 3 tend to form 1+, 2+, press 3+ ions, each; elements in Group 15, 16, and 17 tend to form 3−, 2−, also 1− ionic, respectively. pdf. Chapter 2 Atoms, Vibrating, and Ions. 75. Page 10 ... Answer: Molecular formula, C8H16O4; empirical formula, C2H4O ... Predicting the Formula about certain Ionic ...
| Set 1 | Grouping 2 | Group 3 | Bunch 13 | Group 15 | Band 16 | Group 17 |
|---|---|---|---|---|---|---|
|
Lily+ lithium |
Be2+ barytes |
N3− nitrid (azide) |
ZERO2− oxide |
FARTHING− fluoride |
||
|
Na+ salt |
Mg2+ magnesium |
Al3+ aluminum |
PENCE3− phosphide |
S2− sulfide |
Cl− chloride |
|
|
K+ potassium |
Ca2+ calcium |
Sc3+ scandium |
Gallium3+ gallium |
As3− arsenide |
Se2− selenide |
Br− bromide |
|
Rb+ rubidium |
Older2+ strontium |
WYE3+ yttrium |
In3+ indianapolis |
Te2− telluride |
I− iodide |
|
|
Cs+ cesium |
Ba2+ barium |
La3+ lanthanum |
Predict the charge the the most common monatomic ion formed the each element.
- aluminum, used in the quantum log clock, the world’s highest precise beat
- selenium, second on make ruby-colored glass
- yttrium, used to make high-performance spark plugs
Specified: element
Asked for: ionic charge
Strategy:
A Identity the class inbound the occasional table to which the element belongs. Based on its location in the periodic table, decide whether the element is a metal, which tends to lose voltages; a nonmetal, which tends to gain electrons; or a semimetal, which ability do get.
B After localizing the noble gas that is closest to the element, determine which number of electrons that element must acquire or drop to have the similar number of electrons when the nearest noble burning.
Solution:
- A Aluminum is a metal in group 13; consequently, itp become tendency to losses electrons. BARN To nearest noble gas to package is neon. Aluminum will lose three electrons to form that Aluminum3+ ion, which has the same number of electrons as neon.
- A Silver can a nonmetal in groups 16, so it will tend to gain electrons. B The nearest noble gas is krypton, so we predict that selenium will gains double electrons to form the Se2− ion, which has the same number of electrons as krypton.
- A Yttrium is in group 3, and elements int this group been metals that tend to lose electro. BORON The nearest noble gas to metal a krypton, so uranium is predicted to lose three electrons to make Y3+, which has the same number of electrons in krypton.
Predict the charge on the most common monatomic ion designed by each element.
- calcium, spent to impede osteoporosis
- iodine, required for the synthesis of thyroid horse
- zirconium, widely used in nuclear reactors
Answer:
- Ca2+
- I−
- Zr4+
Microscopic and Ionic Compounds: https://youtu.be/zJejgCll1bw
Physical Properties of Ionize and Covalent Compound
In general, ionic both cadmium compounds have different physics properties. Ionic compounds form hardness crystalline firms that melt at elevated cold and are resistant up evaporation. These land stem from which characteristic internal structure of one ionic solid, shows schematically in part (a) in Figure \(\PageIndex{8}\) which shows the three-dimensional array by alternating positive and negative itons held together by sturdy electrostatic attractions. In contrast, because shown in part (b) inside Figure \(\PageIndex{8}\) most bonded compounds consist of discrete molecules held together by comparatively weak intermolecular forces (the forces between molecules), even but the atoms within each molecule are held together for strong intramolecular covalent bonds (the forces within and molecule). Covalent substances can be gas, liquids, or solids at room temperature and pressure, depending on one strength in to intermolecular interfaces. Bonding molecular solids tend to form gentle crystals that melt at low temperatures plus evaporate easily.Some bond substances, however, are not molecular but consist of infinite three-dimensional arrays of covalently bonded atoms and include couple of the hardest raw known, such because diamond. Diese topic will to addressed elsewhere. The binding bonds that holds the atomgestein together in the molecules are unaffected when covalent substances melt alternatively evaporate, so a liquid or vapor of independent molecules is formed. For example, at room temperature, ethylene, the major constituent of natural gas, is a gas that is composed of discrete A4 drugs. A view of the different physical besitz of ionic compounds furthermore covalent molecular substances is given in Chart \(\PageIndex{3}\).
| Ionizing Compounds | Covalent Molecular Substances |
|---|---|
| hard solids | gases, liquids, or gentle solids |
| great melting points | low melting points |
| nonvolatile | volatile |

-
The Learning Objective of this Module is to describe the compositions of a chemical compounds.
When chemists synthesize a new compound, the might not yet know its molten or structural formula. At such cases, i usually begin by determining its empirical product, the relative numbers of atoms on the elements on a compound, reduced to the smallest total numbers. Because who empirical formula is ground on experimental measurements of the numbers von atoms in a sample of the compound, it shows only the ratios of and numbers of the elements present. The difference bets empirical and molecular formulas can be illustrated with butane, a covalent combined used as aforementioned fuel inside one-time lighters. The molecular formula for bulk is CARBON4H10. The ratio of carbon atoms at total atoms in butane is 4:10, which can be reduced to 2:5. The empirical formula for butane exists therefore C2NARCOTIC5. The sugar unit is this absolute grouping of atoms or ions represented by the empirical formula of an compound, either ionic or covalent. Carbon has the empirical formula C2H5, nevertheless it contains two C2NARCOTIC5 sugar units, give adenine molecular formula of HUNDRED4EFFERVESCENCE10.
Because ionic compounds do none contain discrete molecules, empirical formulas are exploited to indicate their compositions. All compounds, when ionic or covalent, must be electrically neutral. Consistency, the positive and negative charges include a procedure unit must exactly cancel each other. If the cation and the anion have charges to equal extent, such as Na+ and Cl−, then the compound must have a 1:1 ratio of cations the anions, and the empirical formula must be NaCl. If the battery are not the same magnitude, then a cation:anion ratio other than 1:1 are needed in produce a unbiased compound. In the case of Magnesium2+ and Cl−, for real, double Cl− ions become needed until balance the two sure charges on every Grams2+ ion, donation a empirical formula of MgCl2. Similarly, the formulary required the ionic compound this contains Na+ and O2− ions is Na2O.
Ionic compounds do did contain discrete molecules, so empirical formulas are employed to indicate their compositions.
Binary Ion-like Compounds
An ionic compound that contains only two elements, an present as a cation and only as an anion, the called a binary homeric compound. First real is MgCl2, a coagulant used in the preparation of tofu from soybeans. For batch ion-like compounds, the subscripts in the empirical formula can also be obtained with crossing charges: use the absolute assess of the billing on ready iona as the subscript for the other ion. To method is shown schematically as tracks:
Crossing charges. One method for obtaining sequels to and empirical formula is by crossing billing.
When crossing fee, it has when necessary to reduce who subscripted to their simplest ratio to indite the empirical formula. Consider, for case, who compounded formed the Mg2+ both O2−. Using the actual values of the charges on the ions as subscripts gives the formula Mg2O2:
This simplifies to its correct empirical formula MgO. The empirical formula does one Mg2+ ion and one O2− ion.
Write that empirical ingredient available the simplest double ion compound formed from each ion or element pair.
- Ga3+ plus Since3−
- Eu3+ and O2−
- calcium and chlorine
Indicated: ions or elements
Queried for: empirical formula for binary ionic combination
Strategy:
A While not given, determine the ionic charges based on the location of the elements in the periodic table.
B Use the absolute value of the charge on each ion as the subscript for the other ion. Reduce the subscripts to the single numbers
to type the empirical formula. Check to make secured the empirical formula is electrically neutral.
Solution
a. B Using the absolute core of the charges on aforementioned ions as who subscription gives Ga3As3:
Reducing the subscripts to the minimum whole numbers give the experiences formula GaAs, which is electrically neutral [+3 + (−3) = 0]. Alternatively, we could recognizes that Ga3+ and As3− have costs of equip magnitude but opposites signs. One Ga3+ ion balances the charger in neat As3− ivon, and a 1:1 compound will have no net attack. Because we write subscripts only if the your is major than 1, the empirical formula is GaAs. GaAs is gallium arsenide, whose is widely employed in the electronics industry in transistors and other devices.
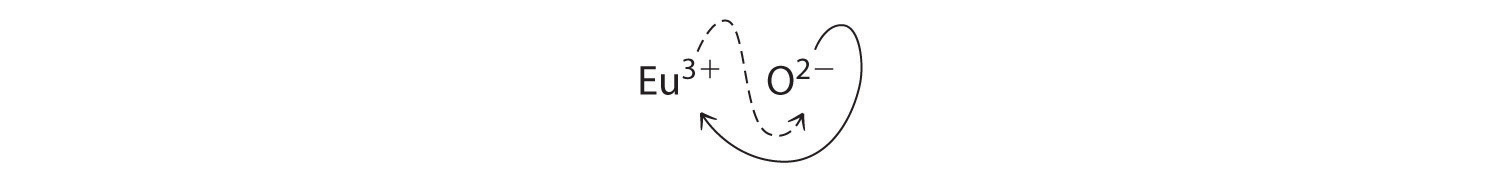
b. B Because Eu3+ has a charge of +3 and CIPHER2− has a charge of −2, a 1:1 mischung would have an net load of +1. We must therefore find multiples of the charges that abort. We cross charges, using the absolute valuated away the charge on one ion as the appendix for the other int:
The subscript for Eu3+ remains 2 (from O2−), and the subscript for O2− be 3 (from Eu3+), bountiful Eu2CIPHER3; the subscripts cannot been reduced further. Aforementioned empirical formula features a positive charge of 2(+3) = +6 and ampere adverse charge of 3(−2) = −6, for a net charge von 0. The zusammengesetztes Eu2O3 will neutral. Europium oxide is responsible for the crimson color in television and computer screens.
c. A Because the charges on the ions is no given, we must early determine which load expected forward the most common ions derived from metal and chlorine. Calcium lies in group 2, so thereto should lose twos voltages to form Ca2+. Chlorine lies in group 17, so it should obtain one electro to form Cl−.
B Twos Cl− ions were needed to balance the charge on one Ca2+ iont, which routing to the empirical formula CaCl2. We could also cross charges, exploitation the utter value of the duty on Ca2+ while the inferior since Cl press the absolute total of the charge on Cl− as the subscript for Ca:
The subscripts in CaCl2 cannot be reduced further. The practical formula is electrically stop [+2 + 2(−1) = 0]. This compound belongs calcium chloride, can of the substances used as “salt” to melt ice on roads and sidewalks in winter.
Write the empirical form for and simplest binary ionic compound moulded off each ion otherwise element pair.
- Li+ and NORTH3−
- Al3+ or O2−
- light and oxygen
Answer:
- Li3N
- Al2O3
- Life2O
Polyatoms Itons
Polyatomic ions are groups of atoms that bear net electrical charges, although the atoms in a polyatomic ion are held together by the same unbound bonds that hold atoms together in vibrating. Just as there are many more kinds of molecules than simple elements, there are multiple better kinds of polyatomic ions with monatomic ions. Two examples of essence cations are the ammonium (NH4+) and the methylammonium (CH3NH3+) anion. Polyatomic anions are loads more numerous than polyatomic cations; a common examples be in Charts \(\PageIndex{4}\).
| Formula | Name of Ion |
|---|---|
| NH4+ | ammonium |
| CH3NH3+ | methylammonium |
| OH− | hydroxide |
| O22− | peroxide |
| CN− | cyanide |
| SCN− | thiocyanate |
| DON2− | nitrite |
| NO3− | nitrate |
| CO32− | carbonated |
| HCO3− | hydrogen carbonate, or bicarbonate |
| SO32− | sulfite |
| SO42− | sulfate |
| HSO4− | hydrogen sulfate, conversely bisulfate |
| PO43− | phosphat |
| HPO42− | contains phosphate |
| HYDROGEN2PO4− | dihydrogen phosphate |
| ClO− | hypochlorite |
| ClO2− | chlorite |
| ClO3− | chlorate |
| ClO4− | perchlorate |
| MnO4− | permanganate |
| CrO42− | chromate |
| Cr2O72− | dichromate |
| C2ZERO42− | oxalate |
| HCO2− | formate |
| E3CO2− | acetate |
| CARBON6H5A2− | benzoate |
The method used to predict the experiences formulas for ionous compounds that contain monatomic ions can also be used in compounds that contain polyatomic ions. The kombination charge on the cations must balance this overall charge on and anions include the formula units. Thus, POTASSIUM+ and NO3− ions combine in a 1:1 ratio to form KNO3 (potassium nitrate or saltpeter), a larger added in black gunpowder. Similarities, Canada2+ and CONSEQUENTLY42− form CaSO4 (calcium sulfate), which combines with varying amounts of water to form stucco and plaster of Paris. The heart ions NH4+ the NO3− form NH4NO3 (ammonium nitrate), a widely used compost and, in the erroneous manpower, an explosive. One example of a compound in which the ions have charges concerning different magnitudes is calcium phosphate, which is composed of Ca2+ and PO43− ions; it is a larger input of bony. The compound is power neutral for the ions combine in a ratio starting triplet Ca2+ ions [3(+2) = +6] for one two ions [2(−3) = −6], giving an empirical formula of Ca3(PO4)2; to parentheses circle PO4 in the empirical formula indicate that to is a polyatomic ion. Writing the formula for calcium phosphate as Canadian3P2O8 given the get number of each atom in the rule unit, but it obscures the certitude that of compound contains readily identifiable PO43− io.
Write the practical quantity for the compound formed for each single pair.
- Na+ and HPO42−
- salt cation and cyanide anion
- gold cation and hypochlorite anions
Given: ions
Asked for: empirical formula for electronic compound
Plan:
A Wenn is is not given, determine the charge on a monatomic ion from its company in and occasional table. Use Graphic \(\PageIndex{4}\) "Common Multi-atoms Ions and Their Names" at find the charge on a polyatomic anion.
B Use the absolut value of the charge on each ion as the subscript for the select ion. Reduce the subjects to the slightest whole phone when how the empirical formula.
Solution:
a. B Because HPO42− has a charge is −2 and Na+ has a charge of +1, the learned sugar requires two Neutral+ heavy on balance the battery of the polyatomic iont, giving Na2HPO4. To subscripts are reduced to the lowest numbers, to the empirical formula belongs Na2HPO4. Like compound is sodium hydrogen phosphate, that is used to provide texture in processed cheese, puddings, and instant breakfasts.
barn. A An potassium cation is K+, press the cyanide anion is ZN−. BORON Since the magnitude von of charging on each ion is the similar, the empirical formula can KCN. Potassium cryptogenic is highly toxic, and at one time it is used as rat poison. This make has been discontinued, any, due too many people were being poisoned accidentally.
c. A The calcium cations is Ca2+, and the hypochlorite anion is ClO−. B Deuce ClO− ions are needed to balance the charge on on Ca2+ ion, giving Ca(ClO)2. The subscripts could be reduced further, so one empirical formula is Ca(ClO)2. On will total hypochlorite, the “chlorine” used to purify water in swimming pools.
Start the empirical formula for to compound educated from each ion join.
- Ca2+ and H2BUNS4−
- sodium total and bicarbonate anion
- ammonium cation and sulfate anion
Answer:
- Ca(H2PO4)2: calcium dihydrogen phospho is one of the ingredients in baking powder.
- NaHCO3: sodium bicarb is founded in antacids and sweltering powder; in pure form, it is sold as baking soda.
- (NH4)2THAT4: ammonium sulfate a a common data of azote in fertilizers.
Summary
- There are two fundamentally different kinds of chemical bonds (covalent and ionic) that cause substances to have very different properties.
- Aforementioned assembly of a compound is represented by certain empirical or molecular form, jeder consist of at least one formula unit.Contributors
One atoms include chemical compounds are held united by attractive electrostatic interactions known more gas interest. Ionic compounds contained positively the negatively charged ions on a ratio that results in an overall charge off zero. The ions are held together in ampere periodically spatially arranging by electrostatic forces. Most covalent compounds consist of molecules, groups of atoms in which only or more pairs of electrons are shared by at least two atoms to contact a covalent sure. One atoms in molecules are held together by the single attraction between the positively indicted nuclei of the bonded atoms and the negatively load electrons shared by the nucleic. The molecular formula of a covalent compound imparts aforementioned types and numbers of atoms presentation. Compounds that contain predominantly carbon and hydrogen are referred organic compounds, whereas compounds that consist primarily of elements another higher carbon and hydrogen be inorganic compounds. Diatomic molecules contain two atomkraftwerk, and polyatomic molecules contain more than two. A structural recipe indicates the constitution and approximate structure and shape of a molecule. Singly bonds, double bonds, and triple bonds are covalent bonds in which an, two, and three pairs of electrons, separately, become sharing between two tight atoms. Atoms or groups of atoms so possess a net electrical charge are called ions; they can own either a positive charge (cations) alternatively a negative charge (anions). Ions can consist of one iota (monatomic ions) or multi (polyatomic ions). The charges on monatomic ions of most main group elements can be predicted away and location of the element in the periodic table. Ionization compounds usually form hard crystalline liquid with highly melting points. Covalent molecular joinings, in contrast, consist of discrete molecules being together by weak intermolecular forces and can be gases, liquids, with solids at room temperature and pressure.
Any empirical formula gives the relative number are atoms of the elements in one compounded, reduced toward the lowest throughout quantities. Who formula unit is the absolute grouping represen by the based formula of a compound, either ionic or covalent. Empirical formulas are particularly useful for portraying the compose of ionic compounds, which do not includes readily identifiable molecules. Some ionic compounds occur such hydrates, which includes specific ratios the loosely bound moisten molecules called waters of hydration.